All Resources
All Resources
Articles, News & Press Releases from Promex.
A fully integrated cGMP manufacturer of FDA compliant Class II / III medical device and biotech assemblies located in the heart of Silicon Valley.
Featured Article
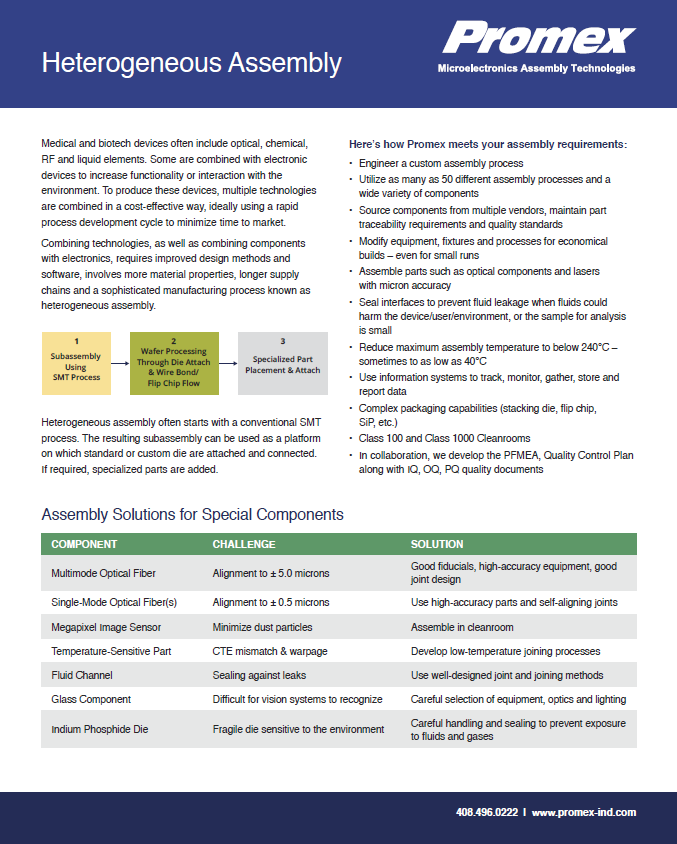
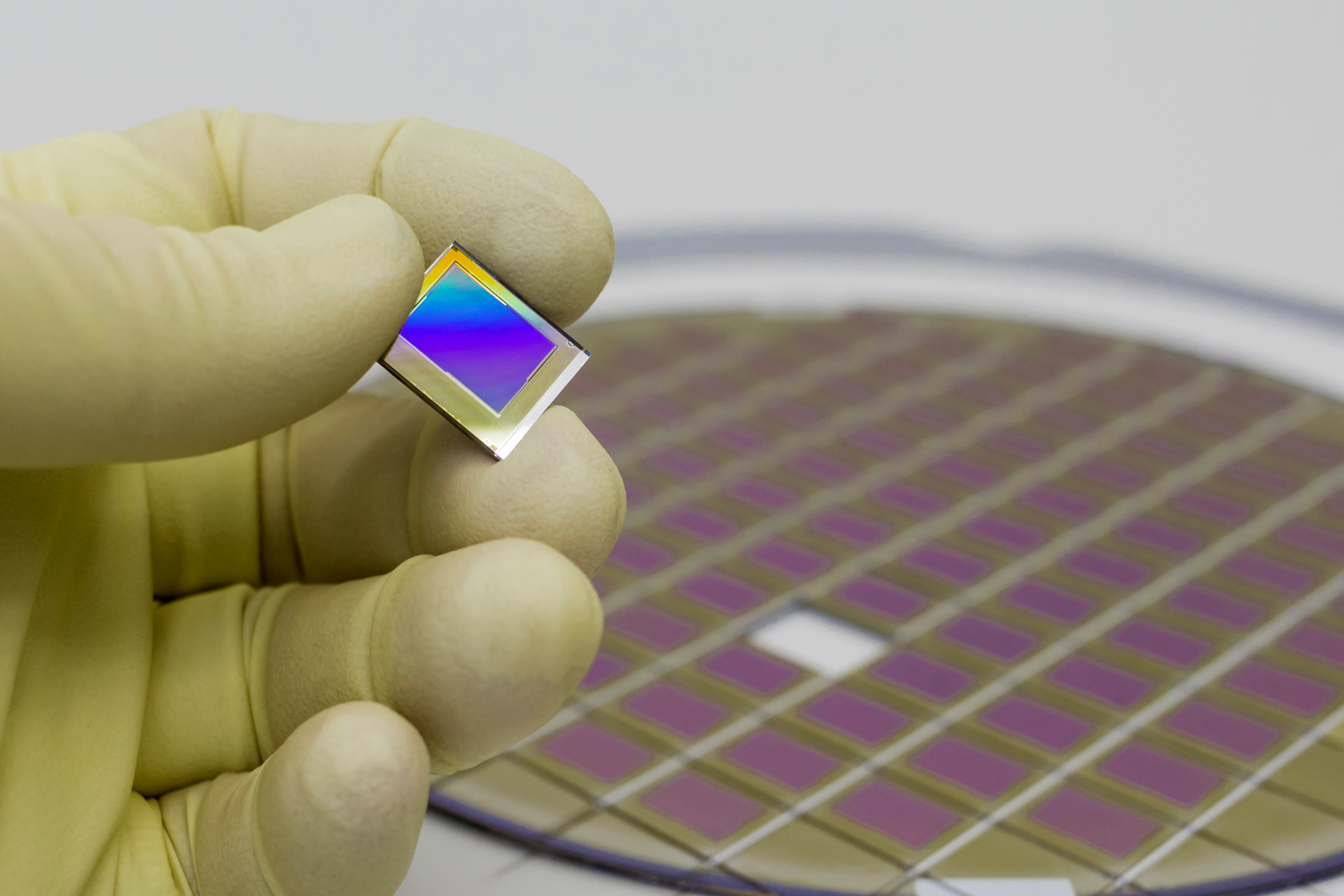
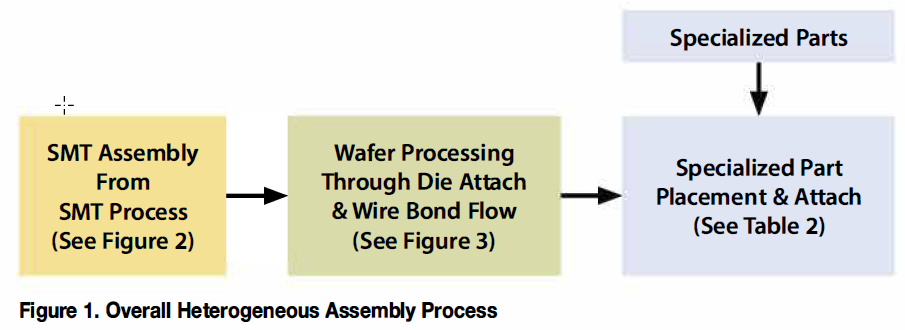
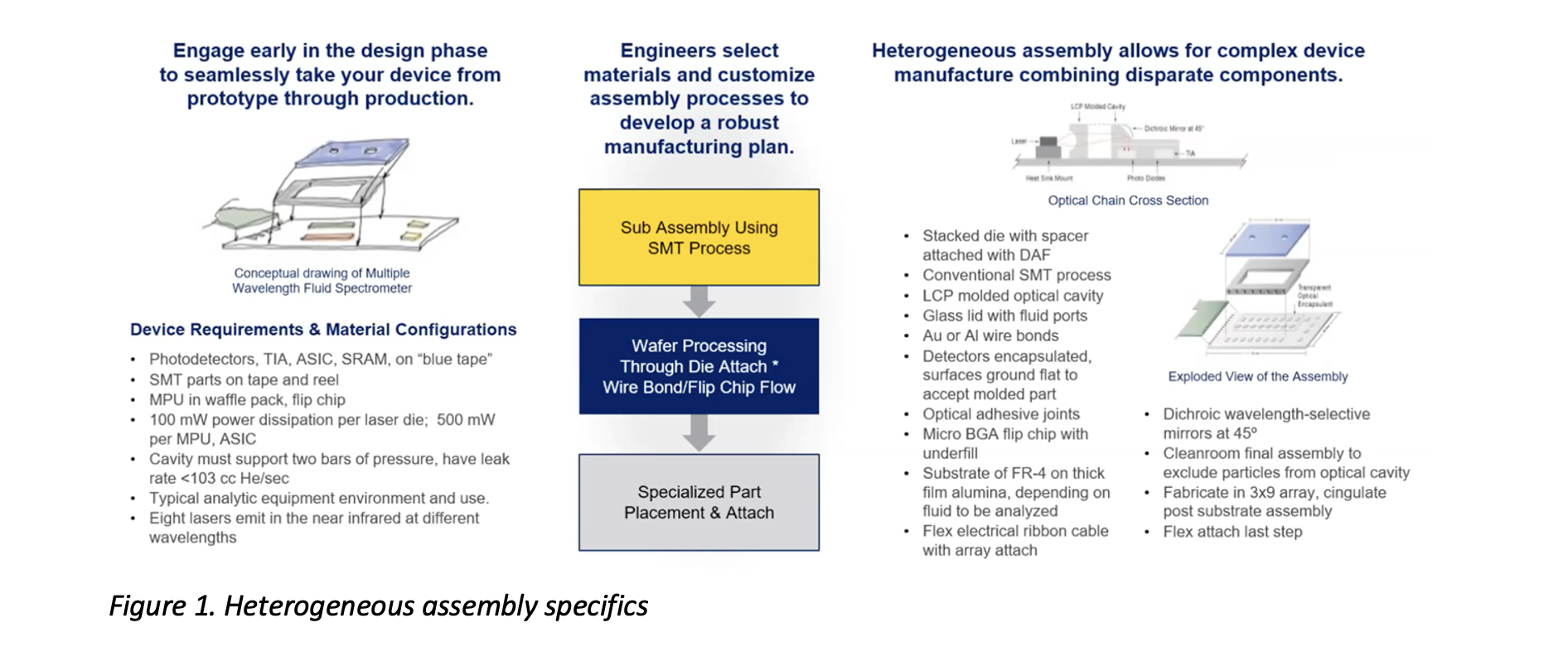
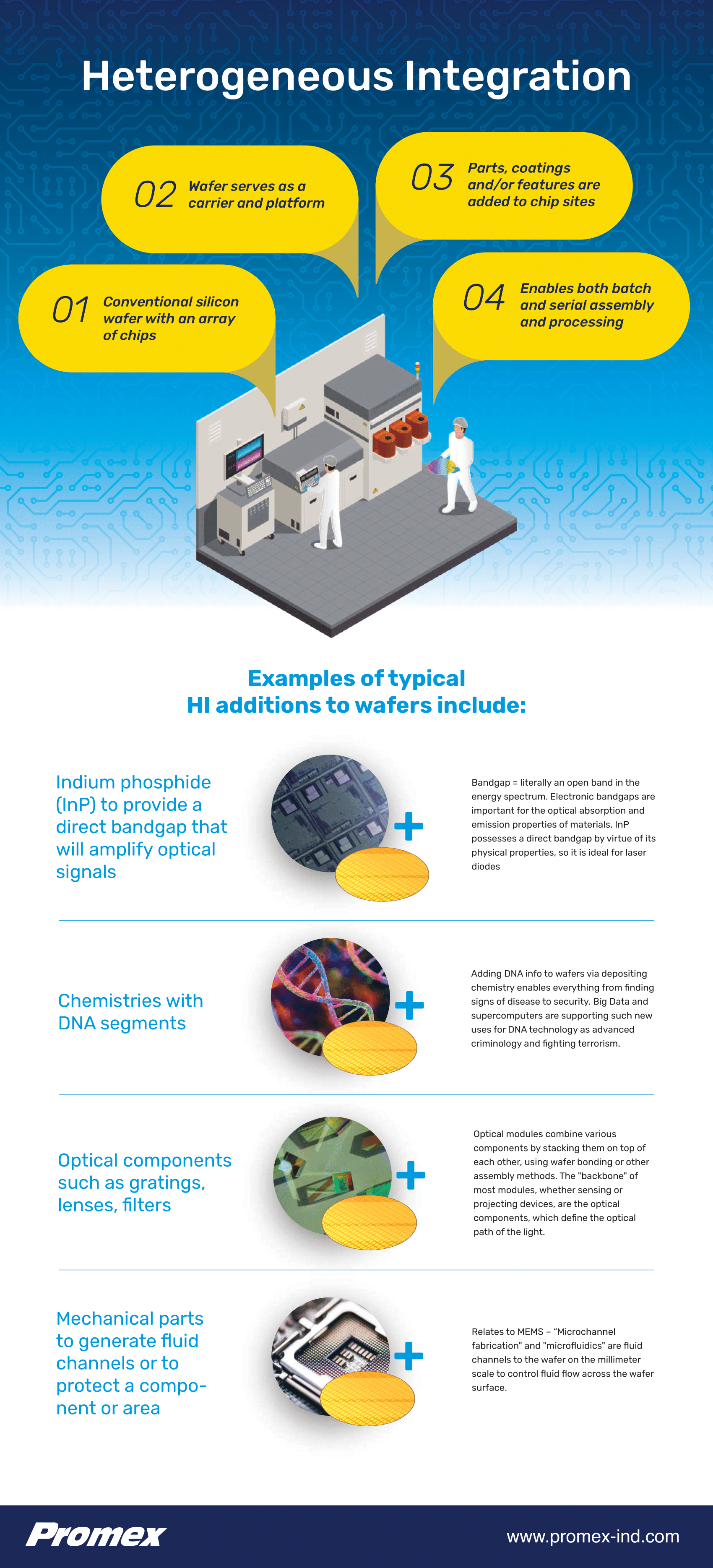
Heterogeneous Assembly Datasheet
Medical and biotech devices often include optical, chemical, RF, and liquid elements. Some are combined with electronic devices to increase functionality or interaction with the environment. To produce these devices, multiple technologies are combined in a...
Tissue Marker Case Study
A short video looking at the fabrication issues for a small radio-frequency tissue marker used in oncology surgeries. Promex specializes in the assembly and fabrication of complex medical devices and biotech instruments and consumables involving customized assembly...
Endoscope Camera Assembly
A short video of how advances in semiconductor assembly and fabrication are re-shaping medical device design. Advances in semiconductor assembly and fabrication are offering medical device designers new options for smaller, more compact, and more sophisticated...
Wafer Singulation
A short video looking at how blade dicing remains the optimal solution for wafer singulation in most cases. Singulation is the process of dicing a silicon wafer into individual units and is a critical step in assembly and packaging. Dicing technology has continued...
Unique Assembly and Production Challenges
Here are some of unique assembly and production challenges we can solve: The smaller the better. We worked with the customer to design an optical Class 3 device that would be inserted into the human body. Requirements included Class 100 assembly and strict...
Advances in Medical Device Design
A short video of how advances in semiconductor assembly and fabrication are re-shaping medical device design:Advances in semiconductor assembly and fabrication are offering medical device designers new options for smaller, more compact, and more sophisticated...
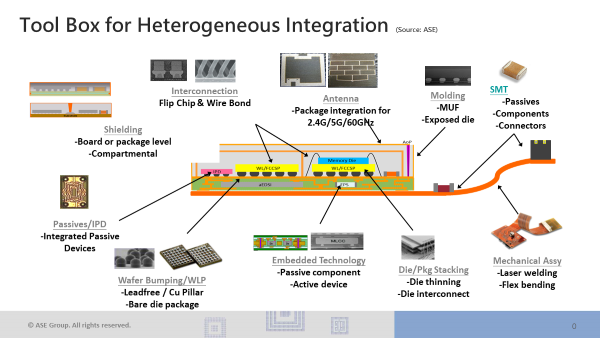
Evolving Heterogeneous Integration Roadmap Highlights Trends
by: Annette Teng, CTO, Promex/Quik-Pak As noted in last month’s blog post, the semiconductor industry has turned its efforts toward the ongoing fleshing out of a roadmap focused on advancing heterogeneous integration (HI). HI integrates separately manufactured...
Heterogenous: It’s More Than Just Integration – It’s Assembly Too
by Promex President and CEO Richard Otte The increased functionality of today's devices is mind-boggling. They go well beyond utilizing just electronics. Optical devices analyze chemicals, toxins, and biologic specimens. Semiconductor devices control and switch...
Heterogenous assembly new? We’ve been doing it for 20 years!
by: Dick Otte, President & CEO Ninety percent of contract manufacturers make devices that are little more than metal boxes with stuff in them. The other ten percent offer specialized processes. Then there is the rare breed of microelectronics assembly services...
Die Attach Film Applications
"Die Attach Film Applications" By Promex CTO Annette TengPublished in MEPTEC Report Winter 2016 Die attach film (DAF) and dicing die attach film (DDAF) have been commercially available since 2000. Epoxy paste adhesives have historically been the sole epoxy material...
Promex Broadens Leadership Team Expertise, Naming Matt Hansen Director of Sales and Business Development
Hansen to leverage breadth of industry experience in targeting new markets SANTA CLARA, Calif., May 15, 2024 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has named...
Browse Resources
Promex Broadens Leadership Team Expertise, Naming Matt Hansen Director of Sales and Business Development
Hansen to leverage breadth of industry experience in targeting new markets SANTA CLARA, Calif., May 15, 2024 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has named...
Exploring the Intricacies of IC Packaging and Assembly Services
Dick Otte, CEO, and Rosie Medina, Vice President, Sales and Marketing, both at QP Technologies, discuss how the company helps its customers with a range of semiconductor technology solutions, covering wafer preparation, advanced assembly and design, IC packaging...
Promex Names David Fromm Chief Operating Officer
SANTA CLARA, Calif. (August 10, 2023) -- Promex, a leader in the assembly of physically small devices that contain semiconductor chips for the medical, biotechnology and communications markets, announced it has named David Fromm its chief operating officer. The...
New York Times: U.S. Focuses on Invigorating Chiplets to Stay Cutting Edge in Tech
For more than 50 years, designers of computer chips mainly used one tactic to boost performance: They shrank electronic components to pack more power onto each piece of silicon. Then more than a decade ago, engineers at the chip maker Advanced Micro Devices began...
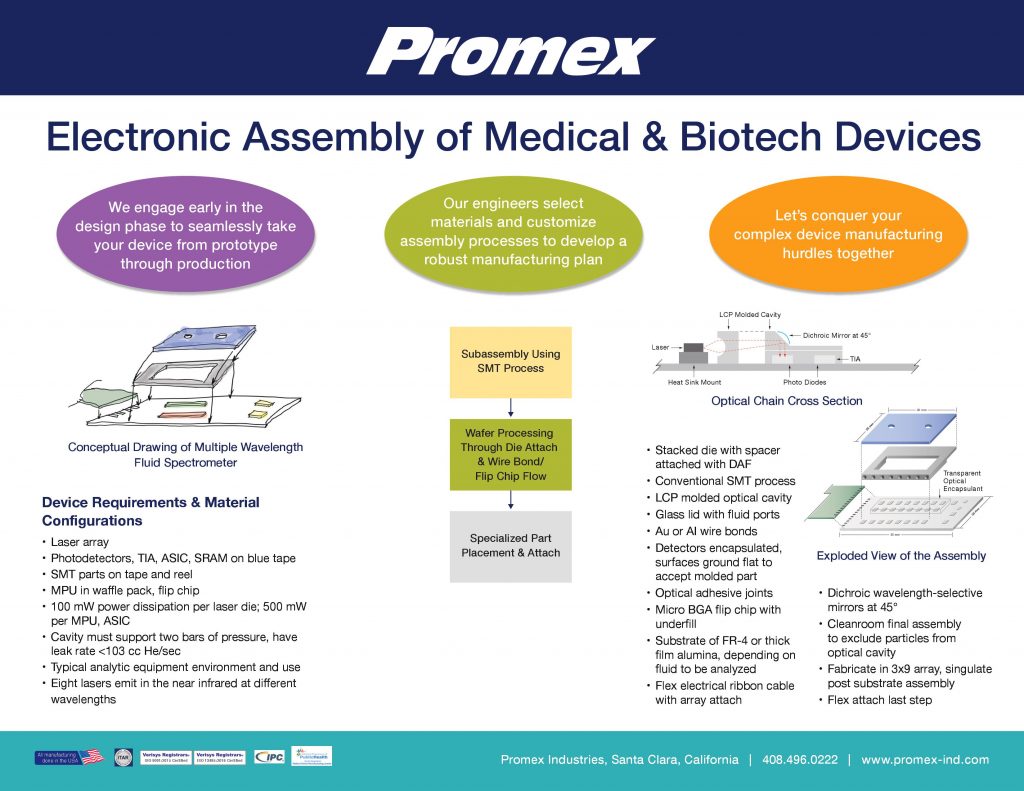
Enabling New Functionality in Medtech and Biotech Devices
By Dick Otte, CEO, Promex Industries Medtech and biotech devices are uniquely suited to benefit from emerging electronic capabilities – specifically, the kind of electronics design, packaging and assembly offerings that are Promex’s specialty. With that said, these...
Promex Bolsters Leadership Team with David Fromm as Vice President of Engineering
Fromm's addition greatly enhances microelectronic component assembly capabilities for medtech and biotech markets. SANTA CLARA, Calif., April 19, 2023 – Promex Industries, a Silicon Valley-based provider of advanced microelectronic component assembly and design...
Promex Further Extends Die-Bonding Proficiencies
Finetech FINEPLACER® Sigma Bonder Targets Range of Application-Specific Projects State-of-the-art Finetech FINEPLACER® sigma advanced sub-micron bonder installed at Santa Clara facility SANTA CLARA, Calif., Feb. 02, 2023 (GLOBE NEWSWIRE) -- Promex Industries, a...
2023 Chiplet Summit
Promex will be exhibiting with Palo Alto Electron at the 2023 Chiplet Summit in San Jose, CA from January 24-26.
iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics
Promex is looking forward to sponsoring the iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics.
Webinar: Heterogeneous Integration Manufacturing
Promex Enables New Functionality in MedTech Devices Combining technologies and components with electronics requires improved design methods, software, more material properties, longer supply chains and a sophisticated manufacturing process known as heterogeneous...
BIOMEDevice Silicon Valley
Promex President & CEO Dick Otte will be participating in the “Biomed Device Manufacturing Panel” discussion at BIOMEDevice 2022 on Tuesday, November 29th from 12:15 - 1 p.m. at Center Stage. Additionally, Promex will be exhibiting at booth 11533 during the...
A Promex Friendsgiving Event
RSVP Here In town for the BIOMEDevice Silicon Valley Conference? Stop by Promex Industries for our Friendsgiving Event. Join us for a tour of our facility while experiencing drinks and savory tapas. We will also be holding a series of raffles that include fabulous...
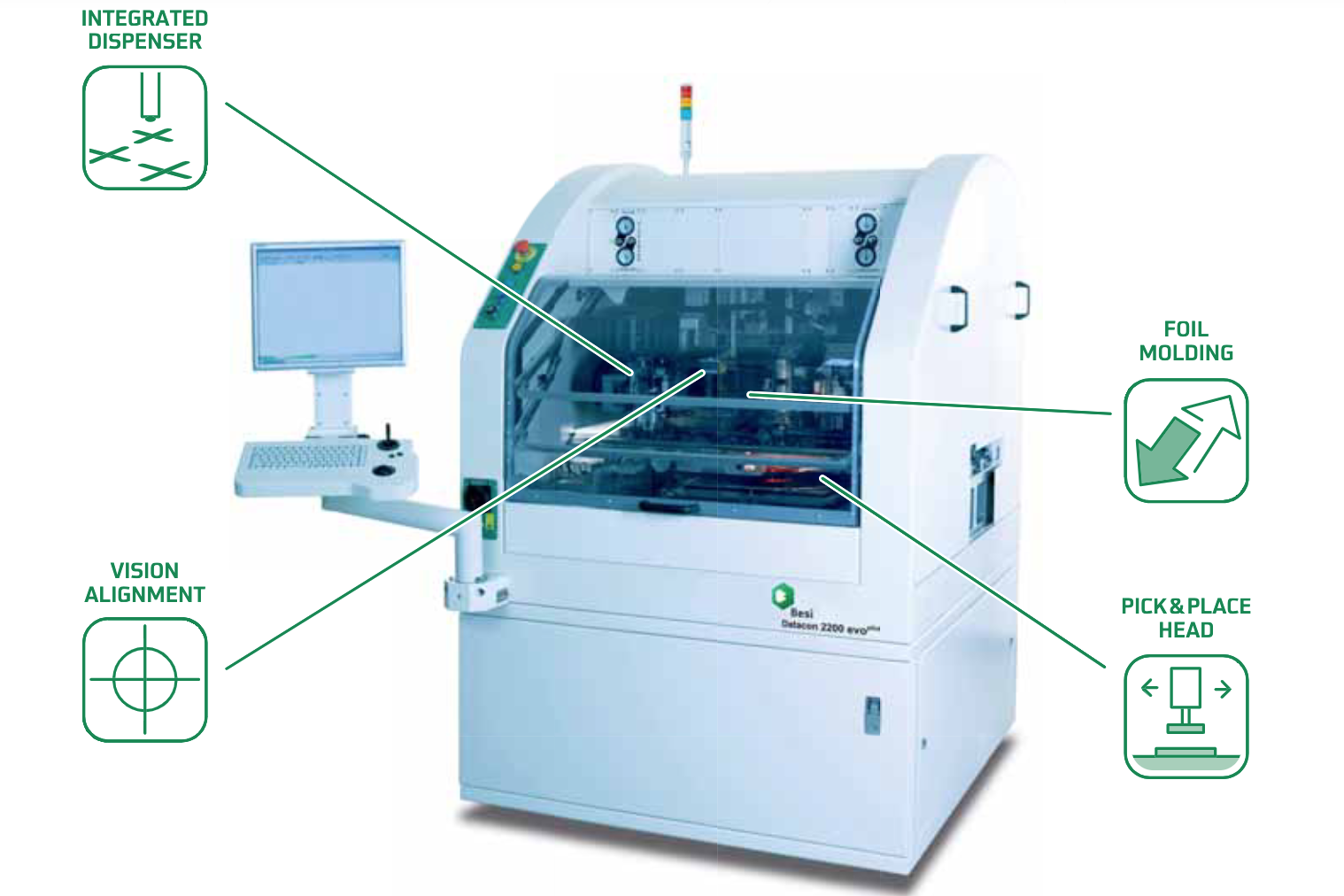
Promex Expands Die Bonding Capacity, Adds New Capabilities
State-of-the-art Besi 2200 evo plus die bonder installed at Santa Clara facility SANTA CLARA, Calif., Oct. 4, 2022 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has...
Heterogeneous Chip Assembly Helps Optimize Medical and Wearable Devices
Heterogeneous integration (HI) has significant implications for the medical, health, and wearables industry. At Promex, we utilize a variety of complex assembly processes to achieve HI for medical and biotech applications. This post will take a closer look at the processes associated with assembling these classes of devices.
Heterogeneous Integration Device Assembly: Key to Enabling Additional Innovations
In Part 1 of this series, we explored the history of medical innovations and how new approaches, like heterogeneous integration (HI), are poised to enable groundbreaking new treatments. Here, we will expand on the role of HI, as well as the assembly challenges for these devices and the importance of working with the right partner to successfully bring new medical devices to market.
Heterogeneous Integration: Fertile Ground for Medical & Biotech Innovation
Heterogenous integration is the newest frontier for the medical and biotech manufacturing services industry to contribute our own innovations by developing the processes to build these unique combinations of electronic, sensing, communication, and “effecting” devices.
Solving the Challenges of MEMS Device Assembly: Part Two
Once you have your MEMS device evaluated, your package chosen and your assembly process flow developed, it’s time to optimize your MEMS project flow. Optimizing your MEMS project flow is essential to achieving reduced costs with high yields. We’ll explore this aspect in part two, examining issues that typically impact a project and key considerations to help stave them off.
Solving the Challenges of MEMS Device Assembly: Part One
Microelectromechanical systems (MEMS) devices have become part of everyday life, as they are being incorporated into an increasingly wide range of applications, from smartphones to industrial instrumentation to medical devices to vehicles.
MEMS & Sensors Technical Congress 2022
Promex President & CEO Dick Otte will present on "Assembly Issues That Are Unique to MEMS Devices," at the 2022 MEMS & Sensors Technical Congress as part of Session 6—Packaging Process Showdown , April 27 at 1:15pm. Learn more About the MEMS & Sensors...
Promex Broadens Leadership Team Expertise, Naming Matt Hansen Director of Sales and Business Development
Hansen to leverage breadth of industry experience in targeting new markets SANTA CLARA, Calif., May 15, 2024 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has named...
Exploring the Intricacies of IC Packaging and Assembly Services
Dick Otte, CEO, and Rosie Medina, Vice President, Sales and Marketing, both at QP Technologies, discuss how the company helps its customers with a range of semiconductor technology solutions, covering wafer preparation, advanced assembly and design, IC packaging...
Promex Names David Fromm Chief Operating Officer
SANTA CLARA, Calif. (August 10, 2023) -- Promex, a leader in the assembly of physically small devices that contain semiconductor chips for the medical, biotechnology and communications markets, announced it has named David Fromm its chief operating officer. The...
New York Times: U.S. Focuses on Invigorating Chiplets to Stay Cutting Edge in Tech
For more than 50 years, designers of computer chips mainly used one tactic to boost performance: They shrank electronic components to pack more power onto each piece of silicon. Then more than a decade ago, engineers at the chip maker Advanced Micro Devices began...
Enabling New Functionality in Medtech and Biotech Devices
By Dick Otte, CEO, Promex Industries Medtech and biotech devices are uniquely suited to benefit from emerging electronic capabilities – specifically, the kind of electronics design, packaging and assembly offerings that are Promex’s specialty. With that said, these...
Promex Bolsters Leadership Team with David Fromm as Vice President of Engineering
Fromm's addition greatly enhances microelectronic component assembly capabilities for medtech and biotech markets. SANTA CLARA, Calif., April 19, 2023 – Promex Industries, a Silicon Valley-based provider of advanced microelectronic component assembly and design...
Promex Further Extends Die-Bonding Proficiencies
Finetech FINEPLACER® Sigma Bonder Targets Range of Application-Specific Projects State-of-the-art Finetech FINEPLACER® sigma advanced sub-micron bonder installed at Santa Clara facility SANTA CLARA, Calif., Feb. 02, 2023 (GLOBE NEWSWIRE) -- Promex Industries, a...
2023 Chiplet Summit
Promex will be exhibiting with Palo Alto Electron at the 2023 Chiplet Summit in San Jose, CA from January 24-26.
iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics
Promex is looking forward to sponsoring the iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics.
Webinar: Heterogeneous Integration Manufacturing
Promex Enables New Functionality in MedTech Devices Combining technologies and components with electronics requires improved design methods, software, more material properties, longer supply chains and a sophisticated manufacturing process known as heterogeneous...
BIOMEDevice Silicon Valley
Promex President & CEO Dick Otte will be participating in the “Biomed Device Manufacturing Panel” discussion at BIOMEDevice 2022 on Tuesday, November 29th from 12:15 - 1 p.m. at Center Stage. Additionally, Promex will be exhibiting at booth 11533 during the...
A Promex Friendsgiving Event
RSVP Here In town for the BIOMEDevice Silicon Valley Conference? Stop by Promex Industries for our Friendsgiving Event. Join us for a tour of our facility while experiencing drinks and savory tapas. We will also be holding a series of raffles that include fabulous...
Promex Expands Die Bonding Capacity, Adds New Capabilities
State-of-the-art Besi 2200 evo plus die bonder installed at Santa Clara facility SANTA CLARA, Calif., Oct. 4, 2022 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has...
Heterogeneous Chip Assembly Helps Optimize Medical and Wearable Devices
Heterogeneous integration (HI) has significant implications for the medical, health, and wearables industry. At Promex, we utilize a variety of complex assembly processes to achieve HI for medical and biotech applications. This post will take a closer look at the processes associated with assembling these classes of devices.
Heterogeneous Integration Device Assembly: Key to Enabling Additional Innovations
In Part 1 of this series, we explored the history of medical innovations and how new approaches, like heterogeneous integration (HI), are poised to enable groundbreaking new treatments. Here, we will expand on the role of HI, as well as the assembly challenges for these devices and the importance of working with the right partner to successfully bring new medical devices to market.
Heterogeneous Integration: Fertile Ground for Medical & Biotech Innovation
Heterogenous integration is the newest frontier for the medical and biotech manufacturing services industry to contribute our own innovations by developing the processes to build these unique combinations of electronic, sensing, communication, and “effecting” devices.
Solving the Challenges of MEMS Device Assembly: Part Two
Once you have your MEMS device evaluated, your package chosen and your assembly process flow developed, it’s time to optimize your MEMS project flow. Optimizing your MEMS project flow is essential to achieving reduced costs with high yields. We’ll explore this aspect in part two, examining issues that typically impact a project and key considerations to help stave them off.
Solving the Challenges of MEMS Device Assembly: Part One
Microelectromechanical systems (MEMS) devices have become part of everyday life, as they are being incorporated into an increasingly wide range of applications, from smartphones to industrial instrumentation to medical devices to vehicles.
MEMS & Sensors Technical Congress 2022
Promex President & CEO Dick Otte will present on "Assembly Issues That Are Unique to MEMS Devices," at the 2022 MEMS & Sensors Technical Congress as part of Session 6—Packaging Process Showdown , April 27 at 1:15pm. Learn more About the MEMS & Sensors...