The Promex Story
The Promex Story
Our objective is simple: solve your complex component assembly challenges. Promex has a long history in Silicon Valley dating from its founding in 1975. The company is privately held and has been under current management since 1995. In 2008 Promex pivoted to provide custom assembly processes for devices that require non-electronic parts that will not tolerate microelectronic assembly processes. This was a fortuitous choice as many contemporary devices combine parts in what is now called Heterogenous Integration.
In summary, Promex builds devices that incorporate semiconductor die and other non-electronic parts in a low-risk path from feasibility prototype development through to full production, all in a single fully integrated U.S. based component assembly facility located in the heart of Silicon Valley.
We’ll Be Your Partner – From Prototype to Production
With a single partner, you can move from fabricating simple combinations of parts and measuring the result to eliminate development risks, through to full production.
Meeting all the necessary requirements and full manufacturing process validation, while still meeting rigorous cost requirements.
A Journey Through Time: Promex’s Microelectronics Assembly Legacy
Promex’s history unfolds through dedication, acquisitions, certifications, and technological leaps, shaping its microelectronics assembly prowess across industries. Scroll down to learn more about our story.
A Journey Through Time: Promex’s Microelectronics Assembly Legacy
Promex’s history unfolds through dedication, acquisitions, certifications, and technological leaps, shaping its microelectronics assembly prowess across industries. Scroll down to learn more about our story.
Founding of Promex
In the heart of Mountain View, CA, Promex was born. Its initial focus was crafting thick-film hybrid circuitry, laying the foundation for its microelectronics expertise.
Relocation To Current Site
The company found a new home on Oakmead Village Drive in Santa Clara, CA, marking a pivotal moment in its journey.
Promex Acquired
A turning point as Promex was embraced by its current owners and welcomed Dick Otte as CEO, charting a path towards innovation.
Class 1000 Cleanroom
The addition of a Class 1000 cleanroom revolutionized chip packaging, while introducing package overmolding capabilities, raising the bar for precision.
ISO 13485
Being certified as meeting the requirements of ISO 13485 marked Promex’s commitment to excellence, steering its focus toward medical and biotech device assembly.
Class 100 Cleanroom
The story continued with the development of a state-of-the-art Class 100 cleanroom production line, symbolizing progress and advancement.
Quik-Pak Acquired
A landmark acquisition of QP Technologies, formerly Quik-Pak, amplified Promex’s capabilities in IC packaging, expanding to their San Diego facility.
Facility Renovation
Reflecting its growth and adaptability, Promex’s Santa Clara Facility underwent a renovation tailored to the needs of medical and biotech services.
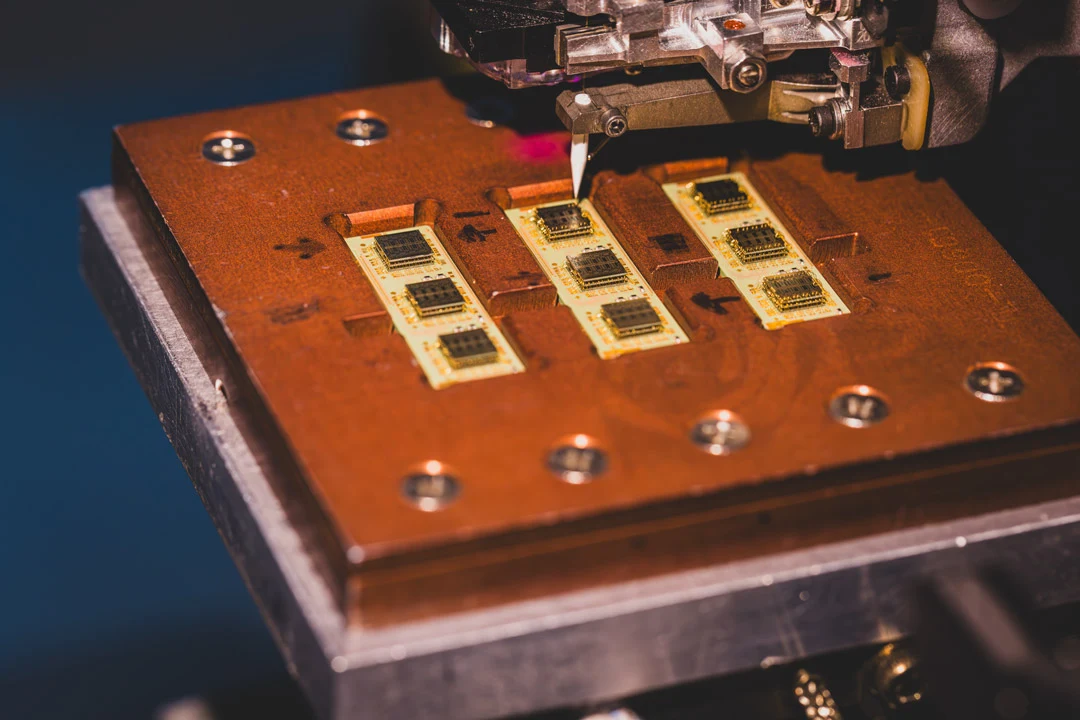
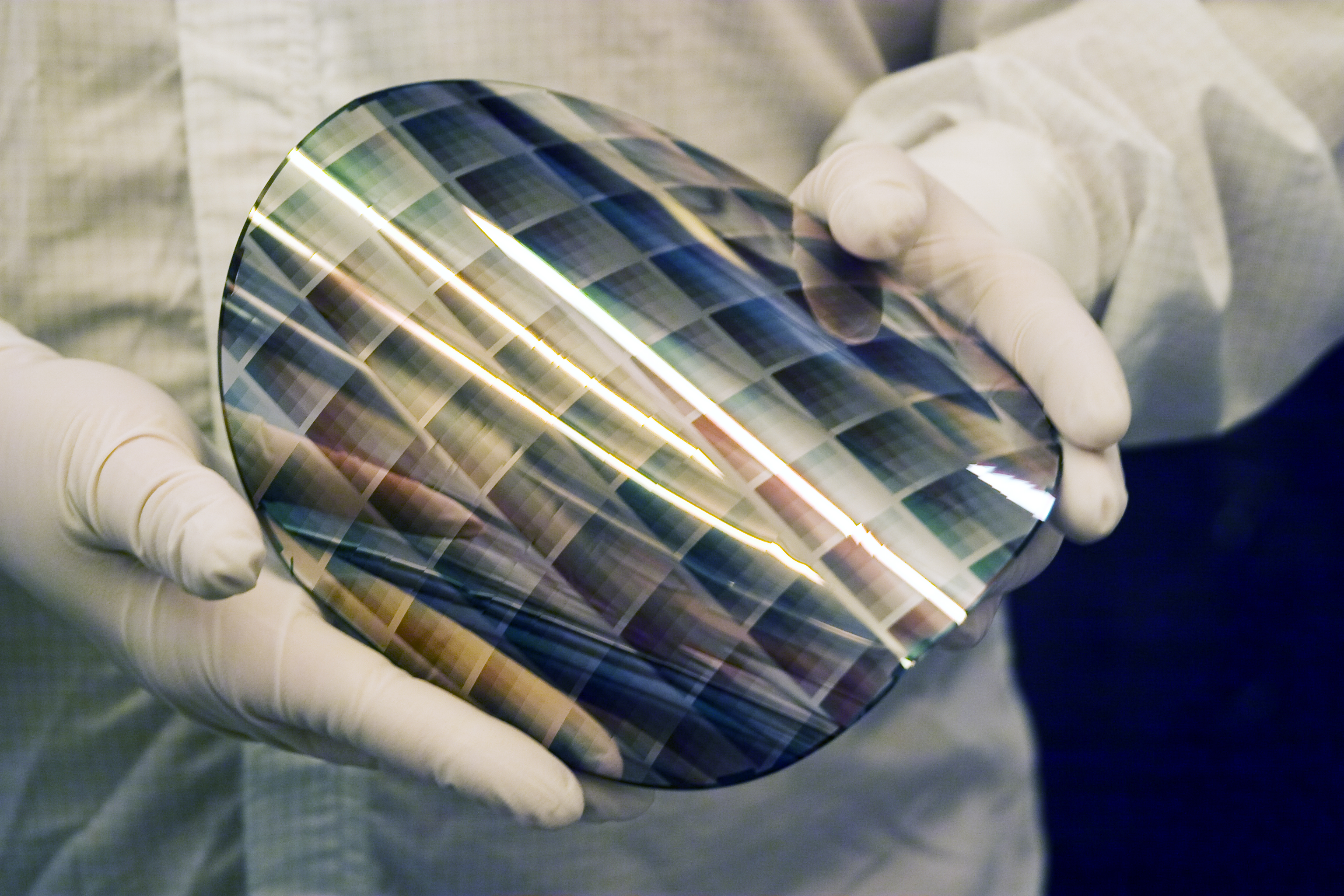




A Note From Our CEO
“It’s not unusual for companies developing complex devices to come to us after other providers failed to meet their IC packaging and assembly requirements. Let us know how we can turn your concept from working prototype to full-scale production.”
Our Facility In Santa Clara, California
We operate a 30,000 sq-ft production facility that is certified as meeting the requirements of ISO 9001:2015 and ISO13485:2016, and ITAR registered. Our SMT lines strictly adhere to the IPC-A-610 standard for electronic assemblies. The Class 100/1000 cleanrooms ensure the quality and cleanliness for specialized microelectronics assemblies.
Our Facility In Santa Clara, California
Our 30,000-sq-ft ISO13485:2016 certified production facility in Santa Clara, California. We are ISO 13485:2016 and ISO 9001:2015 certified and all personnel are certified to the IPC 610 quality standard. Promex is also licensed as a Medical Device Manufacturer by the State of California and is fully ITAR Compliant.
Our Certifications




Typical Clients








Promex Leadership
Contact Information
Promex Industries Inc.
3075 Oakmead Village Dr #0811, Santa Clara, CA 95051
Phone: 408.496.0222
Email: info@promex-ind.com
Searching For A New Career?
Unlock your potential and join our dynamic Promex team. Discover exciting career opportunities that match your skills and aspirations. Your journey starts here. Explore now by clicking the button or email careers@promex-ind.com!

In Need of IC Packaging & Assembly Experts?
Learn more about our affiliated company QP Technologies. QP Technologies provides prototype and small-volume IC packaging and IC assembly services. The company specializes in providing full turn-key solutions that help get your design to market quickly.




