Advanced Microelectronics Assembly – All Under One Roof
Promex Industries is a Silicon Valley-based contract manufacturer with over 50 years of experience delivering precision packaging and assembly for complex microelectronic devices.
Why Promex?
- Integrated Manufacturing Workflow
From wafer processing and die prep to SMT and final box build— everything is done under one roof, by one partner. - Exceptional Quality
Experts in handling complex boards, flex assemblies, and small-form-factor designs. Our ISO-certified quality system ensures robust process control, traceability, and consistency—even for the most complex assemblies. - Flexible Engineering Support
We engage early, adapt quickly, support evolving designs, and reduce design risk through collaboration—no rigid barriers to getting your product built. - Certified & Compliant
ISO 13485:2016 | ISO 9001:2015 | ITAR Registered | Class 100 & 1000 Cleanrooms
When precision, quality, and partnership matters, Promex delivers.
Capabilities
From Prototype To Production
Through collaborative learning with our clients, we design innovative manufacturing process flows that integrate electrical and nonelectrical components into unique assemblies.
Our Phase Gate process will take you from concept to production so you can leverage our expertise in how to design for assembly. We work with your engineering team to ensure your conceptual design is manufacturable and your component assembly can be efficiently achieved.
01 Prototype
Are you involved with early developmental-stage projects requiring short-run prototypes with small-scale fabrication of a few dozen units? We can do rapid prototype builds because of our fully integrated onshore facility located in the heart of Silicon Valley.
02 New Product Introduction
Promex excels in helping bridge the gap between prototype and full-scale production by navigating the complexities of developing production processes for emerging tech. With our experience in assembly, we provide robust support for clients with new product development intricacies.
03 Compliance
We are a fully integrated cGMP manufacturer of FDA-compliant Class II / III medical device assemblies. ISO 13485 certified. We understand the unique demands of FDA compliance and full manufacturing process validation and can help you navigate complexities.
04 Production
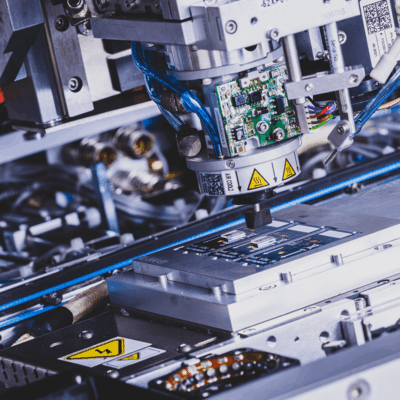
You will have access to conventional microelectronic assembly processes, but we can go beyond these conventional approaches to develop assembly and fabrication processes to accommodate the unique requirements of your device and its components.
Promex Specializes In Innovative Technology
Our technology and engineering expertise combine to offer unique contract production for your project. With our wide range of capabilities all under one roof and your device design needs we work with you to create the production requirements needs. We work with many different industries.
Applications
From Wirebonding Inquiry to Full-Scale Production in Genomics
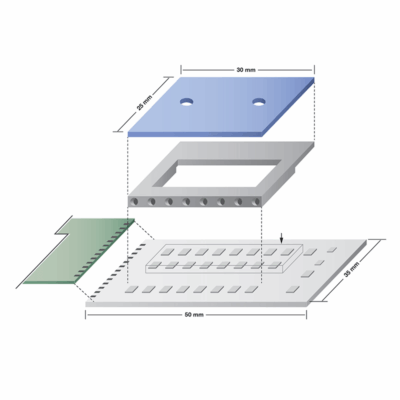
Turning a Sketch into a Production-Ready Module
Clients We Serve
We partner with innovators across medical, aerospace, communications, and emerging technology markets, delivering advanced microelectronics assembly and packaging solutions that meet the highest standards for performance, reliability, and quality.
Promex Is Based In The USA
Founded in 1975, Promex is committed to building smart, capable, miniature devices across many industries, from medical and biotech to automotive and aerospace, and so much more. Let us help you take your concept from prototype to production.