All Resources
All Resources
Articles, News & Press Releases from Promex.
A fully integrated cGMP manufacturer of FDA compliant Class II / III medical device and biotech assemblies located in the heart of Silicon Valley.
Featured Article
White Paper: Advanced Packaging for Medical Microelectronics
Sensors and microelectromechanical systems (MEMS) are enabling more complex designs of miniature devices in healthcare, smart systems, and consumer electronics. To navigate the challenge of building these devices, it’s essential to partner with an expert in...
Browse Resources
Bump Reliability is Challenged By Latent Defects
Thermal stress is a well-known problem in advanced packaging, along with the challenges of mechanical stress. Both are exacerbated by heterogenous integration, which often requires mingling materials with incompatible coefficients of thermal expansion (CTE). Chip...
Promex Further Extends Die-Bonding Proficiencies
Finetech FINEPLACER® Sigma Bonder Targets Range of Application-Specific Projects State-of-the-art Finetech FINEPLACER® sigma advanced sub-micron bonder installed at Santa Clara facility SANTA CLARA, Calif., Feb. 02, 2023 (GLOBE NEWSWIRE) -- Promex Industries, a...
Unknowns And Challenges In Advanced Packaging
Dick Otte, CEO of Promex Industries, sat down with Semiconductor Engineering to talk about unknowns in material properties, the impact on bonding, and why environmental factors are so important in complex heterogeneous packages. What follows are excerpts of that...
The Path To Known Good Interconnects
Chiplets and heterogenous integration (HI) provide a compelling way to continue delivering improvements in performance, power, area, and cost (PPAC) as Moore’s Law slows, but choosing the best way to connect these devices so they behave in consistent and...
2023 Chiplet Summit
Promex will be exhibiting with Palo Alto Electron at the 2023 Chiplet Summit in San Jose, CA from January 24-26.
iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics
Promex is looking forward to sponsoring the iMAPS 8th Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics.
Webinar: Heterogeneous Integration Manufacturing
Promex Enables New Functionality in MedTech Devices Combining technologies and components with electronics requires improved design methods, software, more material properties, longer supply chains and a sophisticated manufacturing process known as heterogeneous...
BIOMEDevice Silicon Valley
Promex President & CEO Dick Otte will be participating in the “Biomed Device Manufacturing Panel” discussion at BIOMEDevice 2022 on Tuesday, November 29th from 12:15 - 1 p.m. at Center Stage. Additionally, Promex will be exhibiting at booth 11533 during the...
A Promex Friendsgiving Event
RSVP Here In town for the BIOMEDevice Silicon Valley Conference? Stop by Promex Industries for our Friendsgiving Event. Join us for a tour of our facility while experiencing drinks and savory tapas. We will also be holding a series of raffles that include fabulous...
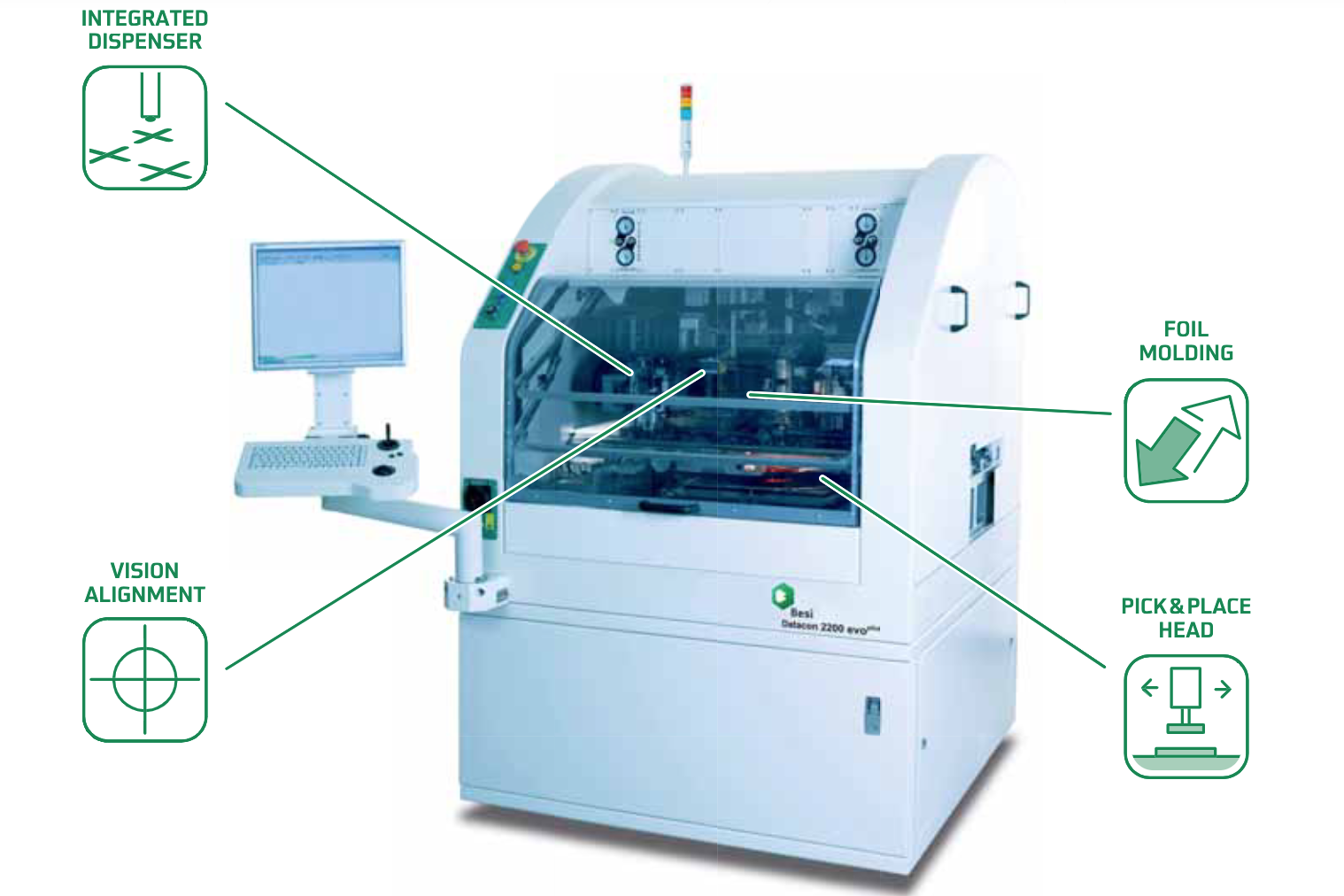
Promex Expands Die Bonding Capacity, Adds New Capabilities
State-of-the-art Besi 2200 evo plus die bonder installed at Santa Clara facility SANTA CLARA, Calif., Oct. 4, 2022 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has...
Heterogeneous Chip Assembly Helps Optimize Medical and Wearable Devices
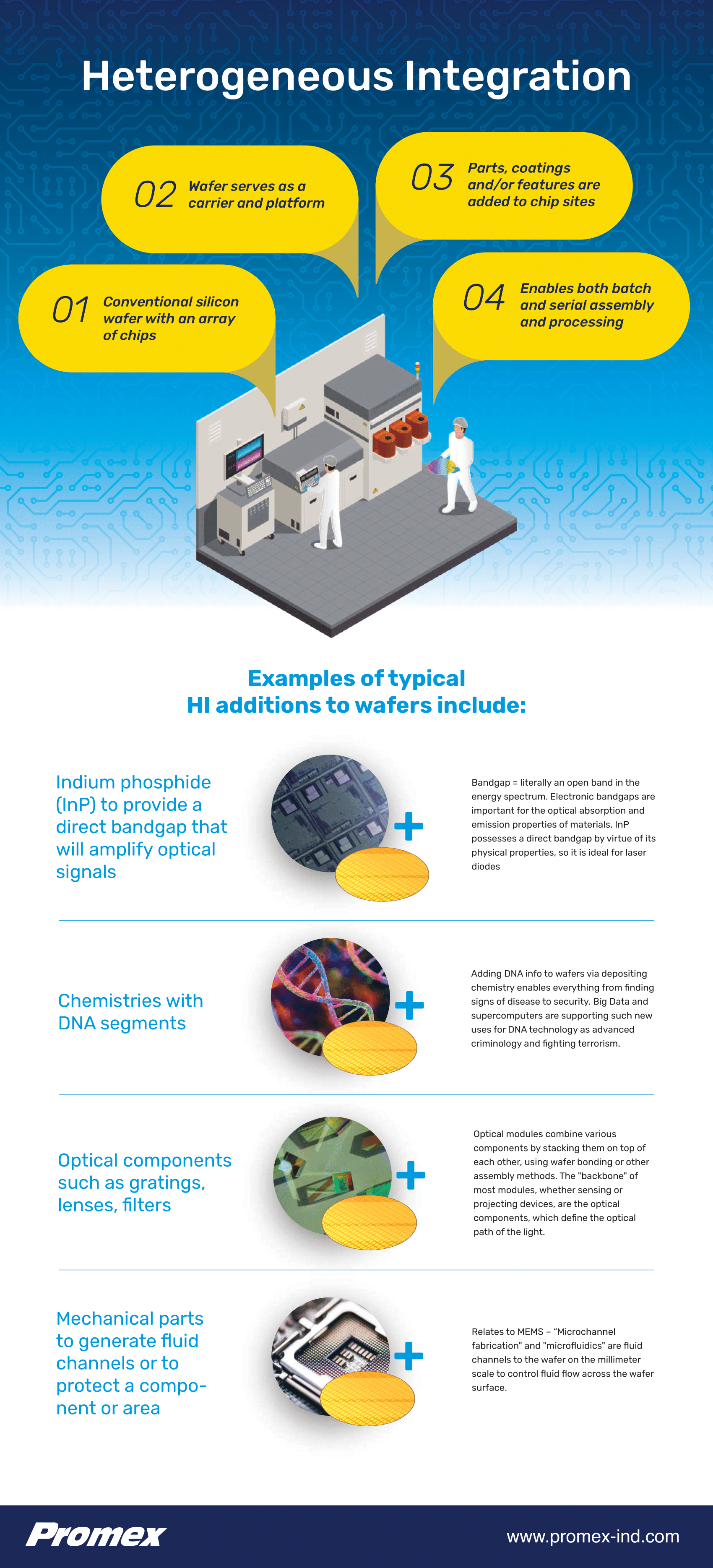
Heterogeneous integration (HI) has significant implications for the medical, health, and wearables industry. At Promex, we utilize a variety of complex assembly processes to achieve HI for medical and biotech applications. This post will take a closer look at the processes associated with assembling these classes of devices.
Heterogeneous Integration Device Assembly: Key to Enabling Additional Innovations
In Part 1 of this series, we explored the history of medical innovations and how new approaches, like heterogeneous integration (HI), are poised to enable groundbreaking new treatments. Here, we will expand on the role of HI, as well as the assembly challenges for these devices and the importance of working with the right partner to successfully bring new medical devices to market.
Heterogeneous Integration: Fertile Ground for Medical & Biotech Innovation
Heterogenous integration is the newest frontier for the medical and biotech manufacturing services industry to contribute our own innovations by developing the processes to build these unique combinations of electronic, sensing, communication, and “effecting” devices.
Solving the Challenges of MEMS Device Assembly: Part Two
Once you have your MEMS device evaluated, your package chosen and your assembly process flow developed, it’s time to optimize your MEMS project flow. Optimizing your MEMS project flow is essential to achieving reduced costs with high yields. We’ll explore this aspect in part two, examining issues that typically impact a project and key considerations to help stave them off.
Solving the Challenges of MEMS Device Assembly: Part One
Microelectromechanical systems (MEMS) devices have become part of everyday life, as they are being incorporated into an increasingly wide range of applications, from smartphones to industrial instrumentation to medical devices to vehicles.
MEMS & Sensors Technical Congress 2022
Promex President & CEO Dick Otte will present on "Assembly Issues That Are Unique to MEMS Devices," at the 2022 MEMS & Sensors Technical Congress as part of Session 6—Packaging Process Showdown , April 27 at 1:15pm. Learn more About the MEMS & Sensors...
MD&M West 2022
Please join us at MD&M West! APRIL 12-14, 2022 Booth 2063 Anaheim Convention Center Book Time with Me Check out the...
A Conversation About Reshoring Advanced Packaging in the U.S.
A 55-minute audio discussion with the CEO of Promex, Dick OtteThe acronyms involving funding for semiconductor manufacturing are flying around Washington. There is the Chips for America Act, focused on re-shoring, The Facilitating American-Built Semiconductors...
Industry Veteran Rosie Medina Appointed as VP of Sales and Marketing for Promex Industries, Inc. Divisions
Industry Veteran Rosie Medina Appointed as VP of Sales and Marketingfor Promex Industries, Inc. Divisions Medina to lead sales and marketing activities for the company’sPromex Medical and Biotech and QP Technologies Entities SANTA CLARA, Calif. – July 20, 2021 –...
No Results Found
The posts you requested could not be found. Try changing your module settings or create some new posts.