All Resources
All Resources
Articles, News & Press Releases from Promex.
A fully integrated cGMP manufacturer of FDA compliant Class II / III medical device and biotech assemblies located in the heart of Silicon Valley.
Featured Article
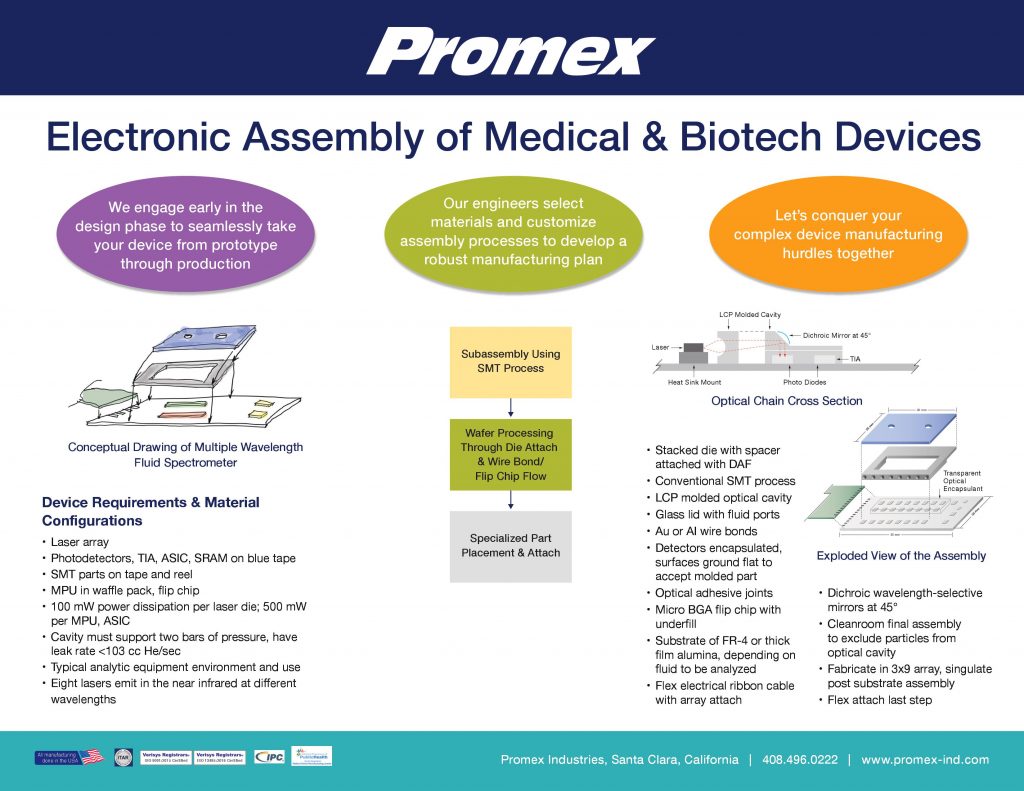
White Paper: Advanced Packaging for Medical Microelectronics
Sensors and microelectromechanical systems (MEMS) are enabling more complex designs of miniature devices in healthcare, smart systems, and consumer electronics. To navigate the challenge of building these devices, it’s essential to partner with an expert in...
Browse Resources
How Die Dimensions Challenge Assembly Processes
Multi-die assemblies are becoming more common and more complex due to technology advancements and market demands, but differing die dimensions are making this process increasingly challenging. In this Semiconductor Engineering article, Dick Otte, CEO of Promex,...
Getting a complex medical device across the finish line is easier when you pass through multiple gates
Medical devices are getting smaller, smarter, and more powerful – much like the evolution of computers. In fact, many of today’s microelectronic-containing medical devices act very much like minicomputers -- sensing, seeing, gathering and sharing data, from both...
3.5D: The Great Compromise
The semiconductor industry is converging on 3.5D as the next best option in advanced packaging, a hybrid approach that includes stacking logic chiplets and bonding them separately to a substrate shared by other components. In this Semiconductor Engineering article,...
Why Small Fab And Assembly Houses Are Thriving
High-volume products get more than their fair share of attention in the semiconductor world, but most chips don’t fit into that category. While a few huge fabs and offshore assembly and test (OSAT) houses process enormous volumes of chips, small fabs and packaging...
How Does Your Med Device Stack Up?
Mechanical tolerance issues can wreak havoc on manufacturing your integrated microelectronic medical device, adding unnecessary costs and delays throughout the product development process and negatively impacting final functional yield. Promex COO and VP of...
Sensor Packaging: Critical MEMS Considerations
Sensor packaging, particularly for microelectromechanical systems (MEMS), is a critical aspect of modern electronics. MEMS developers have demonstrated a variety of innovative microsensors for almost every possible sensing modality including temperature, pressure,...
Promex Industries and QP Technologies Implement Sales/Marketing Reorganization, Promoting Rosie Medina to Promex Senior Vice President and Matt Hansen to QP Technologies Vice President
Two companies heightening focus on individual core competencies to better pursue rapidly expanding business opportunities. SANTA CLARA, Calif., July 29, 2024 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics...
Precision Patterning Options Emerge For Advanced Packaging
The chip industry is ratcheting up investments in advanced packaging as it strives to keep pace with demands for increased functionality and higher performance, including novel patterning technologies that can reduce costs and speed time to market. Promex CEO Dick...
Governments Begin To Shape Metrology Directions
Disruptions to the global semiconductor supply chain caused by the COVID-19 pandemic had a severe impact in nearly every sector of the worldwide economy, and especially the worldwide semiconductor market. In this Semiconductor Engineering article, Dave Fromm, head...
Controlling Warpage In Advanced Packages
Warpage is becoming a serious concern in advanced packaging, where a heterogeneous mix of materials can cause uneven stress points during assembly and packaging, and under real workloads in the field. Promex CEO Dick Otte shared his thoughts on the challenges and...
The Race To Glass Substrates
The chip industry is racing to develop glass for advanced packaging, setting the stage for one of the biggest shifts in chip materials in decades — and one that will introduce a broad new set of challenges that will take years to fully resolve. Dave Fromm, COO at...
Promex Broadens Leadership Team Expertise, Naming Matt Hansen Director of Sales and Business Development
Hansen to leverage breadth of industry experience in targeting new markets SANTA CLARA, Calif., May 15, 2024 – Promex Industries, a Silicon Valley-based provider of advanced design, packaging and microelectronics assembly services, today announced it has named...
What Works Best For Chiplets
The semiconductor industry is preparing for the migration from proprietary chiplet-based systems to a more open chiplet ecosystem, in which chiplets fabricated by different companies of various technologies and device nodes can be integrated in a single package...
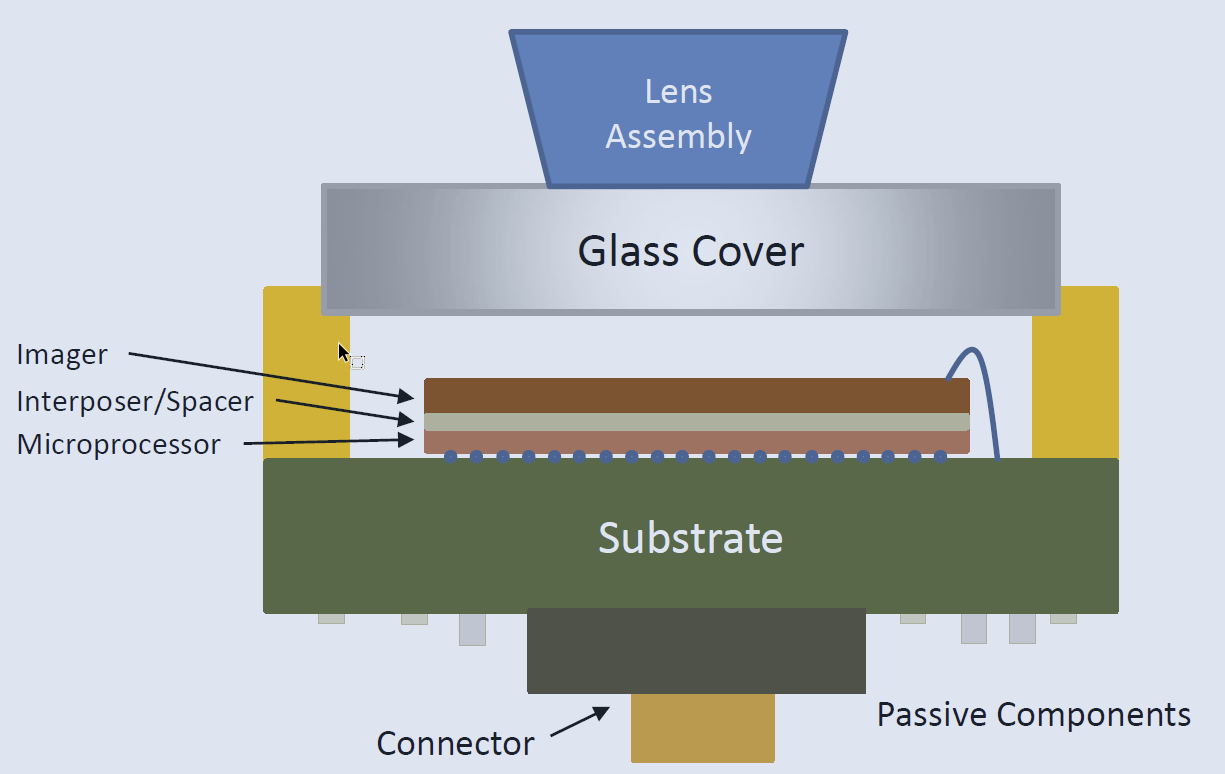
The World’s Tiniest Computers, Medical Devices
With a unique factory setting featuring cutting-edge equipment and meticulous supply chain management, Promex partners with med and biotech companies at all stages in the assembly process, from early prototypes to R&D development and high-volume production....
Silicon Photonics Manufacturing Ramps Up
Circuit scaling is starting to hit a wall as the laws of physics clash with exponential increases in the volume of data, forcing chipmakers to take a much closer look at silicon photonics as a way of moving data from where it is collected to where it is processed...
Choosing adhesives
Medical device designers’ goals have always been enhancing and enriching device functionality while reducing size and cost. This is particularly true as modern devices start acting like complex miniaturized computers for the body, interfaced with microelectronic...
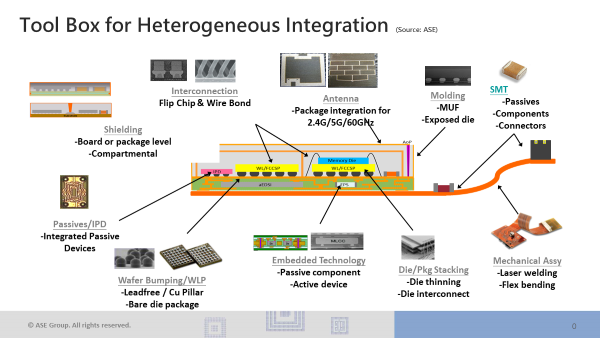
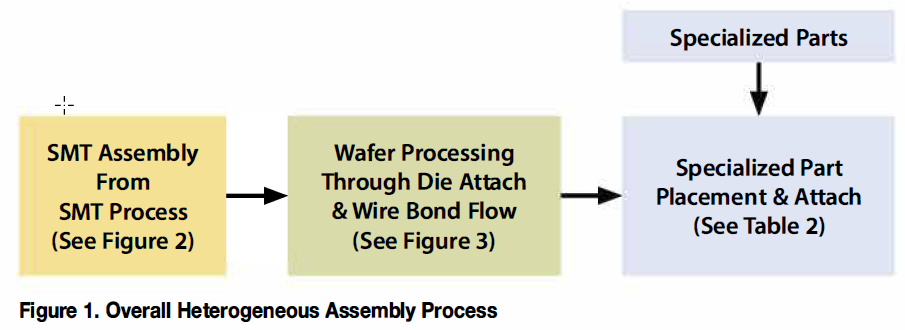
Heterogenous integration packs big innovation into small medical devices
The electronics industry began using the term heterogeneous integration (HI) about five years ago to describe a new approach to building semiconductor devices that would allow for greater density and capability. The legacy approach to making transistors, lines, and...
The Miniaturization Revolution in Electronics
In the realm of electronics and semiconductors, a silent revolution is underway, one marked not by the grandeur of size, but by the subtlety of miniaturization. This long-term trend toward smaller, more compact devices is not just a technological feat; it’s a...
Navigating Heat In Advanced Packaging
The integration of multiple heterogeneous dies in a package is pivotal for extending Moore’s Law and enhancing performance, power efficiency, and functionality, but it also is raising significant issues over how to manage the thermal load. Dave Fromm, COO and vice...
Wafer Singulation
A short video looking at how blade dicing remains the optimal solution for wafer singulation in most cases. Singulation is the process of dicing a silicon wafer into individual units and is a critical step in assembly and packaging. Dicing technology has continued...
Unique Assembly and Production Challenges
Here are some of unique assembly and production challenges we can solve: The smaller the better. We worked with the customer to design an optical Class 3 device that would be inserted into the human body. Requirements included Class 100 assembly and strict...
Advances in Medical Device Design
A short video of how advances in semiconductor assembly and fabrication are re-shaping medical device design:Advances in semiconductor assembly and fabrication are offering medical device designers new options for smaller, more compact, and more sophisticated...
Evolving Heterogeneous Integration Roadmap Highlights Trends
by: Annette Teng, CTO, Promex/Quik-Pak As noted in last month’s blog post, the semiconductor industry has turned its efforts toward the ongoing fleshing out of a roadmap focused on advancing heterogeneous integration (HI). HI integrates separately manufactured...
Promex Awarded CA FDB Medical Device Manufacturing License
Microelectronics Assembly Technology Provider Exhibits June 25-27 at Sensors 2019 (Booth 1229) in San Jose, CA and at IMAPS Advanced SiP in Monterey, CA June 20, 2019 – Promex is now licensed to manufacture Class II and Class III medical devices by The State of...
Promex Industries to Showcase Heterogeneous Integration Expertise for Medical Devices at IMAPS Workshop for Advanced Packaging of Medical Microelectronics
SANTA CLARA, Calif. – January 27, 2020 – Promex Industries, a biotech microelectronics manufacturer specializing in heterogeneous integration of key subsystems for medical, diagnostic, and life sciences devices, announced its participation in this week’s technical...
Promex CTO Annette Teng Receives EPS Regional Contributions Award for Outstanding Leadership, Impact on Programs and Growth
Santa Clara, Calif. – PR NEWSWIRE May 30, 2018 — Promex, a provider of innovative IC packaging and heterogeneous assembly solutions for medical, biotech, and sensor-based microelectronic devices, announces that Promex Chief Technology Officer Annette Teng has been...
Promex Explores Disruptive New Generation of Flexible Hybrid Electronics
Electronics on Everything” may soon be more than just a promise with the advent of flexible hybrid electronics. FHE leverages the low cost of printed plastic film substrates and the performance of semiconductor devices to create a new category of electronics. What...
Promex CEO Richard Otte Presents at IEEE VLSI Test Symposium
The IEEE VLSI Test Symposium tapped Promex President and CEO Richard Otte for a presentation on the status, needs, and potential solutions for testing photonic devices and products. Held earlier this month in San Francisco, the symposium explored emerging trends...
Heterogenous: It’s More Than Just Integration – It’s Assembly Too
by Promex President and CEO Richard Otte The increased functionality of today's devices is mind-boggling. They go well beyond utilizing just electronics. Optical devices analyze chemicals, toxins, and biologic specimens. Semiconductor devices control and switch...
Heterogenous assembly new? We’ve been doing it for 20 years!
by: Dick Otte, President & CEO Ninety percent of contract manufacturers make devices that are little more than metal boxes with stuff in them. The other ten percent offer specialized processes. Then there is the rare breed of microelectronics assembly services...
Developing new medical devices? Start with a free design review for reliability and manufacturability.
by: Rosie Medina, Director Sales & Marketing Medical devices and biotech systems often involve people, animals, bacteria, viruses, and other living entities lacking standard electrical connectors. Instead, they use sensors, actuators, antennas, and probes to...
Even if all you’ve got for your new 5G product is a proof of concept, we can help accelerate the process
by: Dick Otte, President & CEO Annual revenues for 5G services and products are estimated to reach $250 billion by 2025. No wonder manufacturers are racing to be first to market. These new function-rich 5G products will incorporate more sensors and devices and...
Die Attach Film Applications
"Die Attach Film Applications" By Promex CTO Annette TengPublished in MEPTEC Report Winter 2016 Die attach film (DAF) and dicing die attach film (DDAF) have been commercially available since 2000. Epoxy paste adhesives have historically been the sole epoxy material...
Promex Industries Acquires Quik-Pak, a Division of Delphon
Santa Clara, CA, April 1, 2015: Promex Industries, announced today the acquisition of San Diego based Quik-Pak, a division of Delphon. Quik-Pak will retain its San Diego, CA location and operate as a division of Promex. The combined entities are now able to offer...
Advances in Medical Device Package Manufacturing
“Advances in Medical Device Package Manufacturing” by Former Promex Chief Technology Officer Dr. Edward S. Binkley. Presented at the MEPTEC Packaging Symposium, October 2014.Here's a downloadable version (.pdf) of the paper:[pdf-embedder...
Development Status of an OCCAM Electronic Assembly Method
"Development Status of an OCCAM Electronic Assembly Method" by Promex CEO Richard F. Otte and Former Chief Technology Officer Dr. Edward S. Binkley. Published June 2014.Here's a downloadable version (.pdf) of the paper:[pdf-embedder...
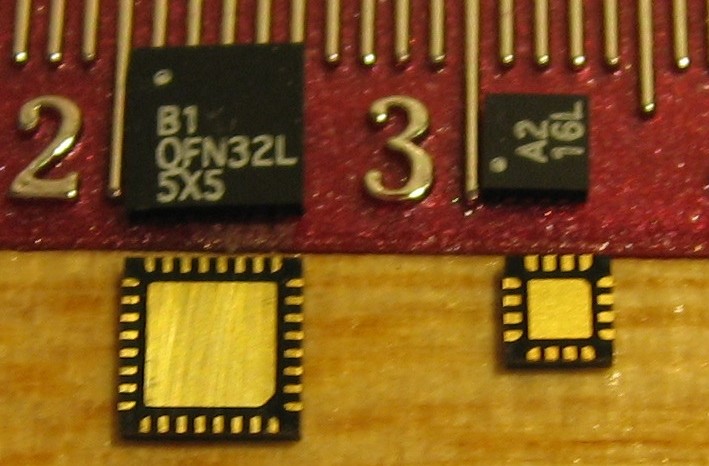
A Simplified QFN Package Characterization Technique
"A Simplified QFN Package Characterization Technique” by Promex CEO Richard F. Otte and by Dr. Eric Bogatin and Trevor Mitchell, Bogatin Enterprises. Published August 2010. This technique was used to characterize the electrical parasitics (R, C, and L vs frequency)...