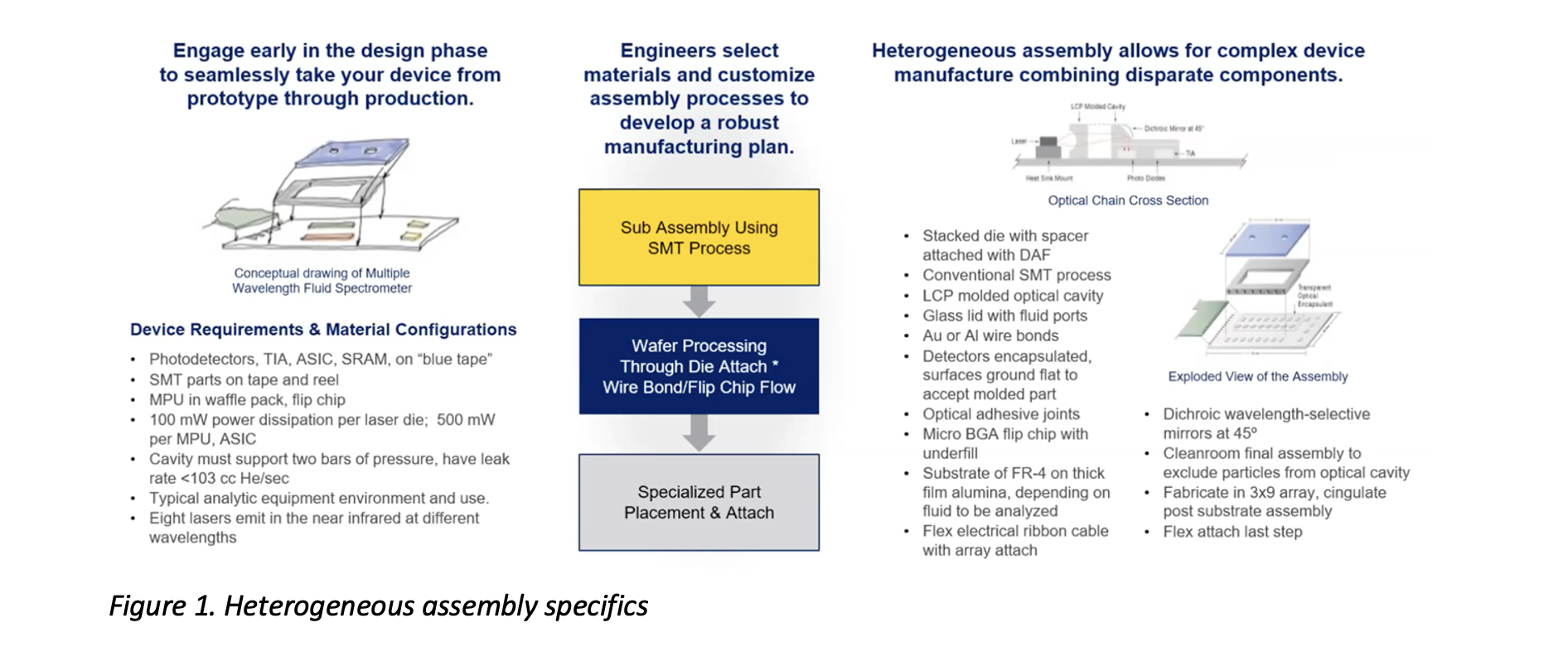
All Resources
All Resources
Articles, News & Press Releases from Promex.
A fully integrated cGMP manufacturer of FDA compliant Class II / III medical device and biotech assemblies located in the heart of Silicon Valley.
Featured Article
White Paper: Advanced Packaging for Medical Microelectronics
Sensors and microelectromechanical systems (MEMS) are enabling more complex designs of miniature devices in healthcare, smart systems, and consumer electronics. To navigate the challenge of building these devices, it’s essential to partner with an expert in...
Browse Resources
Powerful Sensors for the Eye
Advances in integrated microelectronics have enabled a revolution in ever smaller and more powerful medical sensors. One such use is for detecting pressure in the eye. InjectSense has invented an implantable sensor that detects direct dynamic pressure in the eye...
Heterogeneous Integration Finding Its Footing
Semiconductor Engineering sat down to discuss heterogeneous integration with Dick Otte, president and CEO of Promex Industries; Mike Kelly, vice president of chiplets/FCBGA integration at Amkor Technology; Shekhar Kapoor, senior director of product management...
Wirebonding Is Here To Stay
Few technologies in semiconductor manufacturing have stood the test of time as steadfastly as wirebonding. This process, which involves electrically connecting semiconductor devices to their packages, has been a cornerstone of the electronics industry since the...
Navigating thermally induced dynamics when developing miniaturized medical devices
Some complexities of integrating miniaturized components, such as microelectronics, into increasingly small medical devices are obvious. Examples include the precision required to position and align components with the requisite accuracy, or the...
Building Better Bridges In Advanced Packaging
The increasing challenges and rising cost of logic scaling, along with demands for an increasing number of features, are pushing more companies into advanced packaging. And while that opens up a slew of new options, it also is causing widespread confusion over what...
How to Build Better Medical Microelectronics
Advances in semiconductors, materials, power, and more are enabling electronics to miniaturize—including medical electronics. Design News asked Dave Fromm, Promex’s chief operating officer, a few questions about engineering microelectronics for medical devices....
Right Sizing Implantable Pressure Sensors
More uses for implantable sensors are being discovered every day. One such capability is pressure detection, including the detection of intra-ocular pressure, helpful for treating glaucoma. In this article for Medical Device & Diagnostic Industry, Promex's Dave...
Exploring the Intricacies of IC Packaging and Assembly Services
Dick Otte, CEO, and Rosie Medina, Vice President, Sales and Marketing, both at QP Technologies, discuss how the company helps its customers with a range of semiconductor technology solutions, covering wafer preparation, advanced assembly and design, IC packaging...
Promex Names David Fromm Chief Operating Officer
SANTA CLARA, Calif. (August 10, 2023) -- Promex, a leader in the assembly of physically small devices that contain semiconductor chips for the medical, biotechnology and communications markets, announced it has named David Fromm its chief operating officer. The...
Goals of Going Green
The chip industry is stepping up efforts to be seen as environmentally friendly, driven by growing pressure from customers and government regulations. In this Semiconductor Engineering article, Dick Otte, CEO of Promex, discussed how the company is working towards...
Enabling New Functionality In Medtech And Biotech Devices
Medtech and biotech devices are uniquely suited to benefit from emerging electronic capabilities – specifically, the kind of electronics design, packaging and assembly offerings that are Promex’s specialty. With that said, these markets present a variety of...
Challenges Grow For Creating Smaller Bumps For Flip Chips
New bump structures are being developed to enable higher interconnect densities in flip-chip packaging, but they are complex, expensive, and increasingly difficult to manufacture. Jeff Schaefer, senior process engineer at Promex Industries, shared insights into...
New York Times: U.S. Focuses on Invigorating Chiplets to Stay Cutting Edge in Tech
For more than 50 years, designers of computer chips mainly used one tactic to boost performance: They shrank electronic components to pack more power onto each piece of silicon. Then more than a decade ago, engineers at the chip maker Advanced Micro Devices began...
Enabling New Functionality in Medtech and Biotech Devices
By Dick Otte, CEO, Promex Industries Medtech and biotech devices are uniquely suited to benefit from emerging electronic capabilities – specifically, the kind of electronics design, packaging and assembly offerings that are Promex’s specialty. With that said, these...
U.S. Focuses on Invigorating ‘Chiplets’ to Stay Cutting-Edge in Tech
Chiplets, a way to design chips for higher performance, has become a key prong of U.S. industrial policy. But pushing for more of this activity domestically is challenging. Dick Otte, CEO of Promex Industries, shared his insights into this challenge with The New...
3D Structures Challenge Wire Bond Inspection
Adding more layers in packages is making it difficult, and sometimes impossible, to inspect wire bonds that are deep within the different layers. Jeff Schaefer, senior process engineer of Promex Industries, shared insights into this challenge in this Semiconductor...
Promex Bolsters Leadership Team with David Fromm as Vice President of Engineering
Fromm's addition greatly enhances microelectronic component assembly capabilities for medtech and biotech markets. SANTA CLARA, Calif., April 19, 2023 – Promex Industries, a Silicon Valley-based provider of advanced microelectronic component assembly and design...
Big Changes Ahead In Power Delivery, Materials, And Interconnects
When it comes to device interconnects, it’s hard to beat copper. Its low resistivity and high reliability have served the industry exceedingly well as both on-chip interconnect and wires between chips. But in logic chips, with interconnect stacks rising into the...
Mini-Consortia Forming Around Chiplets
Mini-consortia for chiplets are sprouting up across the industry, driven by demands for increasing customization in tight market windows and fueled by combinations of hardened IP that have been proven in silicon. Dick Otte, CEO of Promex Industries, shared his...
Managing Thermal-Induced Stress In Chips
Heterogeneous integration and increasing density at advanced nodes are creating some complex and difficult challenges for IC manufacturing and packaging. Chip Greely, vice president of engineering at Promex, offered insights into this issue to Semiconductor...
No Results Found
The posts you requested could not be found. Try changing your module settings or create some new posts.